Major metropolitan hospitals
- Princess Alexandra, QLD - 39%
- Fremantle, WA - 52%
Major regional hospitals
- Royal Hobart, TAS - 61%
- Coffs Harbour, NSW - 65%
Large hospitals
- Calvary Mater (Newcastle) - 47%
- Repatriation General, SA - 83%

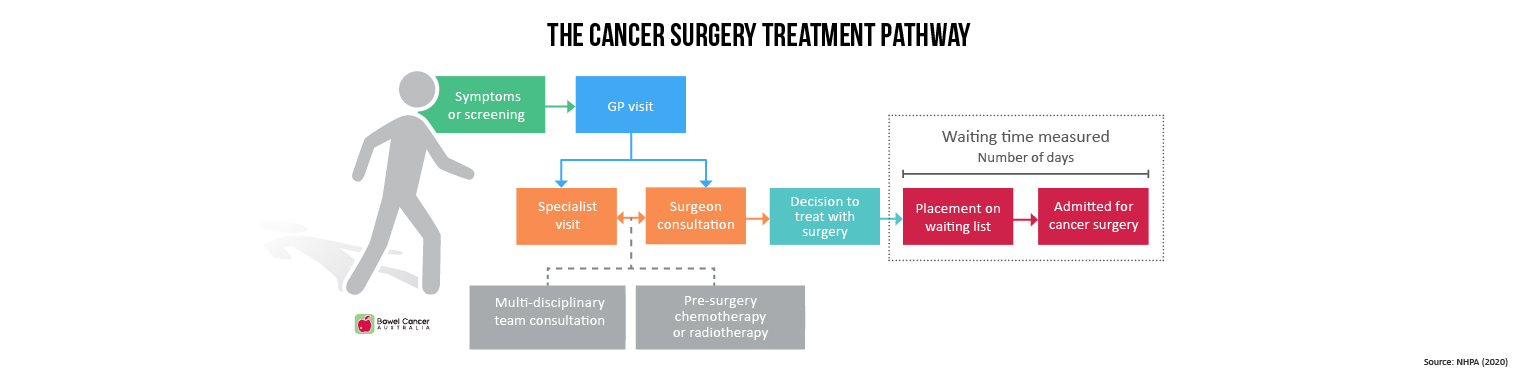
A course of radiotherapy is a series of one or more external beam radiotherapy treatments prescribed by a radiation oncologist. A patient can receive more than one course of radiotherapy at the same time (courses that are simultaneous or overlap).
The waiting time is the number of days from when a patient is ready to be treated with radiotherapy in the opinion of the treating specialist (‘ready for care’) until the day the patient first receives radiotherapy treatment - that is, the number of days between the ready-for-care date and the radiotherapy start date.
Reported waiting times include non-working days (such as weekends or public holidays) and other days on which a service was not able to provide services (such as when key staff are unavailable or where there has been equipment failure).
Other waiting periods—such as the time between when a person contacts their GP and their first appointment with a medical oncologist, and the time between receipt of the patient’s first referral to a radiation oncologist to the date of that patient’s first consultation with a radiation oncologist are not included in calculating the radiotherapy waiting times.
The ready-for-care date is set by the treating specialist and takes into account things such as the need for prior treatment or post-operative healing. If a patient is not ready for care on this date for personal reasons, the ready-for-care date will be set at a later time, when a patient states they are ready.
Treatment may be delayed due to waiting times in pre-treatment imaging or testing, treatment service availability, staff shortages, equipment breakdown, or even a lack of available accommodation for a patient travelling for treatment.
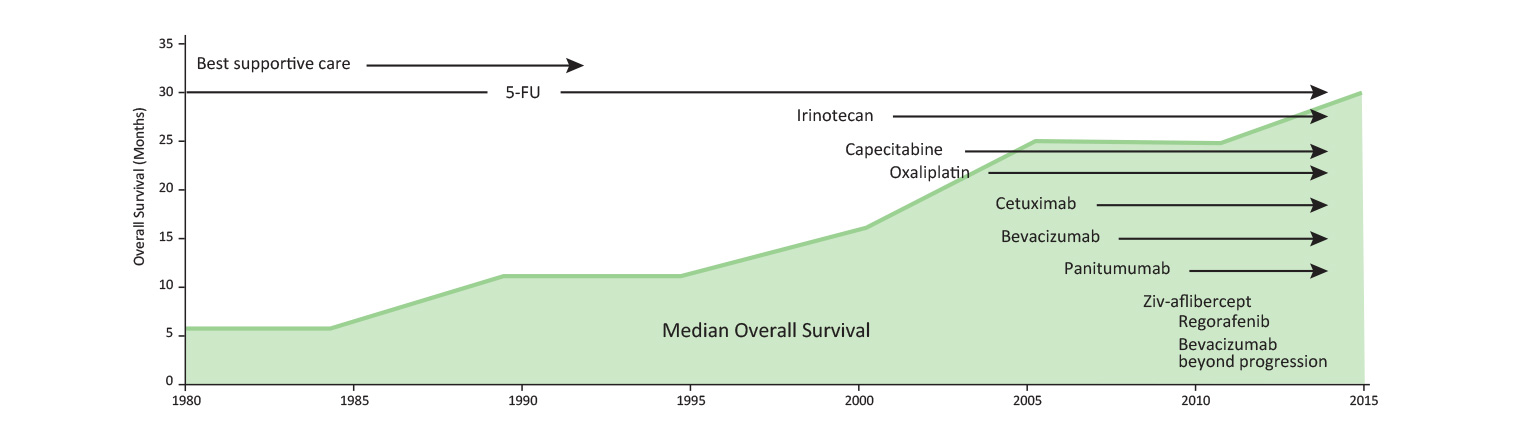
Patients with metastatic bowel cancer want to live as full a life as possible, for as long as possible, and will actively seek out new treatments.
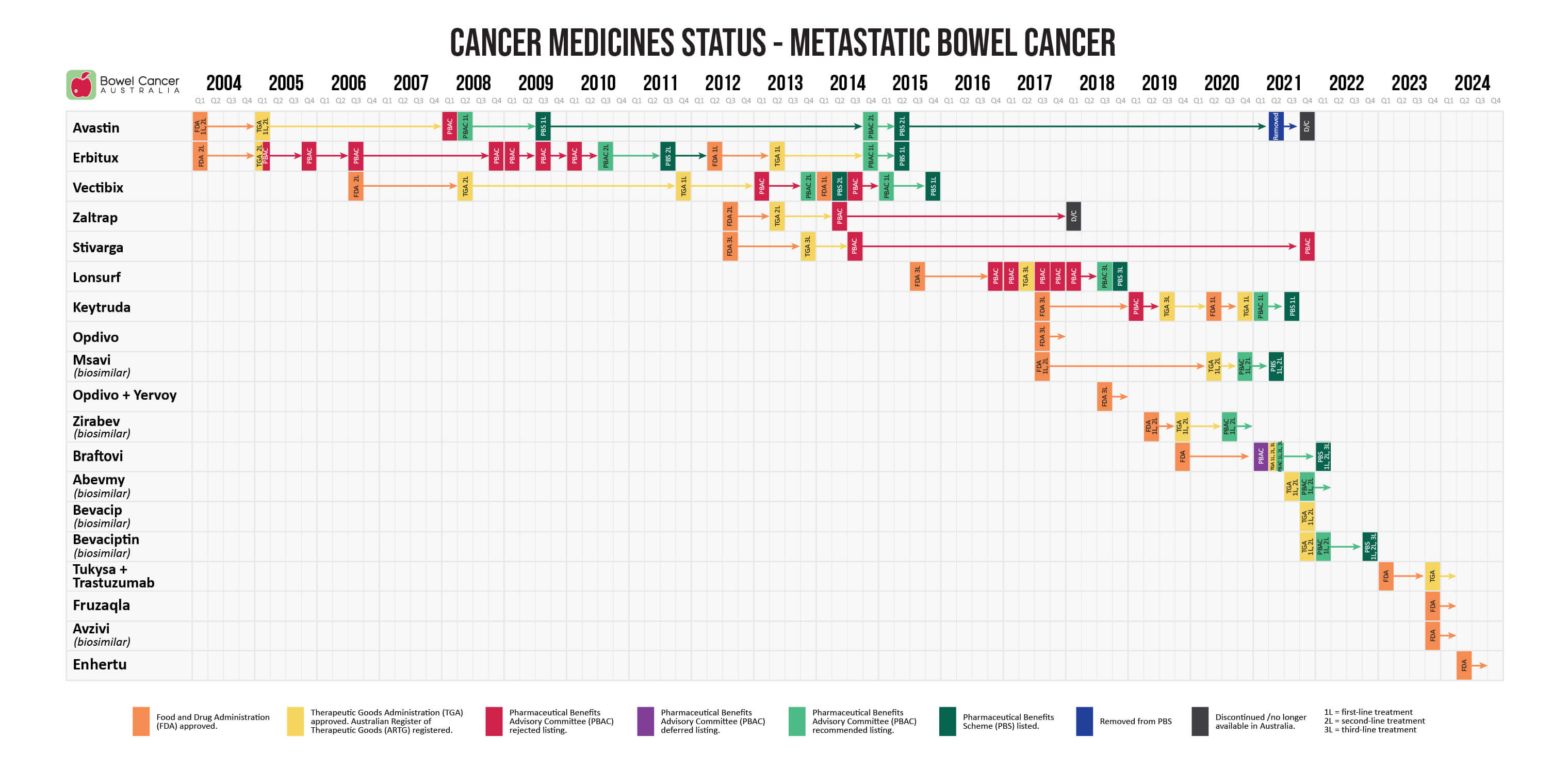

- April 2024 - the US Food and Drug Administration (FDA) granted accelerated approval to Enhertu (trastuzumab deruxtecan) for adult patients with unresectable or metastatic HER2-positive solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options.

- December 2023 - the US Food and Drug Administration (FDA) granted approval of Avzivi (bevacizumab-tnjn), a biosimilar brand of Avastin, in combination with IV fluorouracil-based chemotherapy for first- or second-line treatment of metastatic bowel cancer, and in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for second-line treatment of metastatic bowel cancer that has progressed on a first-line regimen containing bevacizumab.

- November 2023 - the US Food and Drug Administration (FDA) granted approval to Fruzaqla (fruquintinib) for the treatment of adult patients with metastatic bowel cancer who received prior fluoropyrimidine -, oxaliplatin -, and irinotecan-based chemotherapy, an anti-VEGF therapy, and if RAS wild-type and medically appropriate, an anti-EGFR therapy.

- November 2023 - the Therapeutic Goods Administration (TGA), via the provisional approval pathway, registered Tukysa (tucatinib) in combination with trastuzumab on the Australian Register of Therapeutic Goods (ARTG), for the treatment of adult patients with RAS wild-type HER2-positive unresectable or metastatic bowel cancer that has progressed following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
- January 2023 - the US Food and Drug Administration (FDA) granted accelerated approval to Tukysa (tucatinib) in combination with Herzuma (trastuzumab), a biosimilar brand of Herceptin, for RAS wild-type HER2-positive unresectable or metastatic bowel cancer that has progressed following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.

- March 2022 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Bevaciptin, as a biosimilar brand of Avastin, for the first- or second-line treatment of patients with metastatic bowel cancer on the Pharmaceutical Benefits Scheme (PBS) as a subsidised treatment option.
- November 2021 - the Therapeutic Goods Administration (TGA) evaluated and registered Bevaciptin, as a biosimilar brand of Avastin, on the Australian Register of Therapeutic Goods (ARTG), for the first- or second-line treatment of patients with metastatic bowel cancer.

- November 2021 - the Therapeutic Goods Administration (TGA) evaluated and registered Bevacip, as a biosimilar brand of Avastin, on the Australian Register of Therapeutic Goods (ARTG), for the first- or second-line treatment of patients with metastatic bowel cancer.
 Abevmy (biosimilar)
Abevmy (biosimilar)- November 2021 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Abevmy, as a biosimilar brand of Avastin, for the first- or second-line treatment of patients with metastatic bowel cancer on the Pharmaceutical Benefits Scheme (PBS) as a subsidised treatment option.
- September 2021 - the Therapeutic Goods Administration (TGA) evaluated and registered Abevmy, as a biosimilar brand of Avastin, on the Australian Register of Therapeutic Goods (ARTG), for the first- or second-line treatment of patients with metastatic bowel cancer.

Braftovi
- May 2021 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Braftovi in combination with Erbitux for the targeted treatment of patients with BRAF V600E-variant metastatic bowel cancer who have received prior systemic therapy. This followed MSAC's support at its 31 March - 1 April 2021 meeting to amend the descriptor for MBS item 73338 to include BRAF V600 testing.
- May 2021 - the Therapeutic Goods Administration (TGA) registered Braftovi in combination with Erbitux on the Australia Register of Therapeutic Goods (ARTG), for the treatment of adult patients who have metastatic bowel cancer with a BRAF V600E mutation as detected by a validated test, and who have received prior systemic therapy.
- March 2021 - the Pharmaceutical Benefits Advisory Committee (PBAC) deferred the listing of Braftovi (encorafinib) until the Medical Services Advisory Committee (MSAC) decides to list the BRAF V600 biomarker test on the Medical Benefits Schedule (MBS). If MSAC decides to list the biomarker test, the PBAC would support an expedited process for reconsideration to align any PBAC recommendation for listing Braftovi with the circumstances supported by MSAC.
-
April 2020 - the US Food and Drug Administration (FDA) approved Braftovi in combination with Erbitux for the treatment of adult patients with metastatic bowel cancer with BRAF V600E mutation after prior therapy.
-
December 2019 - the US Food and Drug Administration (FDA) accepted and granted priority review for Braftovi in combination with Erbitux with or without Mektovi, for the treatment of patients with metastatic bowel cancer with BRAF V600E mutation following one or two lines of therapy.

Zirabev (biosimilar)
- July 2020 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Zirabev, as a biosimilar brand of Avastin, for the first- or second-line treatment of patients with metastatic bowel cancer on the Pharmaceutical Benefits Scheme (PBS) as a subsidised treatment option.
- November 2019 - the Therapeutic Goods Administration (TGA) evaluated and registered Zirabev, as a biosimilar brand to Avastin, on the Australian Register of Therapeutic Goods (ARTG), for the first- or second-line treatment of patients with metastatic bowel cancer.
- June 2019 - the US Food and Drug Administration (FDA) approved Zirabev, as a biosimilar to Avastin, for the first- or second-line treatment of patients with metastatic bowel cancer.

Mvasi (biosimilar)
- December 2020 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Mvasi, as a biosimilar brand of Avastin, for the first- or second-line treatment of patients with metastatic bowel cancer on the Pharmaceutical Benefits Scheme (PBS) as a subsidised treatment option.
- June 2020 - the Therapeutic Goods Administration (TGA) registered Mvasi, as a biosimilar brand of Avastin, on the Australian Register of Therapeutic Goods (ARTG), for the first- or second-line treatment of patients with metastatic bowel cancer.
- September 2017 - the US Food and Drug Administration (FDA) approved Mvasi, as a biosimilar brand of Avastin, for the first- or second-line treatment of patients with metastatic bowel cancer.

Opdivo + Yervoy
-
July 2018 - the US Food and Drug Administration (FDA) granted accelerated approval to Yervoy for use in combination with Opdivo for the treatment of patients 12 years of age and older, with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic bowel cancer that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
-
July 2017 - the US Food and Drug Administration (FDA) granted accelerated approval to Opdivo for the treatment of patients 12 years of age and older with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic bowel cancer that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.

Keytruda
- March 2021 - the the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Keytruda on the Pharmaceutical Benefits Scheme (PBS) as a first-line treatment option in adults with previously untreated unresectable or metastatic deficient mismatch repair (dMMR) bowel cancer (mCRC).
- December 2020 - the Therapeutic Goods Administration (TGA) registered Keytruda on the Australia Register of Therapeutic Goods (ARTG), for the first-line treatment of previously untreated patients with unresectable or metastatic bowel cancer that is microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR).
- June 2020 - the US Food and Drug Administration (FDA) approved Keytruda as a monotherapy for the first-line treatment of patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) bowel cancer.
- July 2019 - the Therapeutic Goods Administration (TGA), via the provisional approval pathway, registered Keytruda on the Australian Register of Therapeutic Goods (ARTG), for the treatment of adult and paediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) bowel cancer that has progressed following treatment with fluoropyrimidine, oxaliplatin , and irinotecan.
- March 2019 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Keytruda on the Pharmaceutical Benefits Scheme (PBS) as a second-line treatment option for locally advanced (unresectable) or Stage IV (metastatic) bowel cancer with deficient mismatch repair (dMMR).
The PBAC considered that insufficient evidence was provided in the submission to evaluate the efficacy and safety of Keytruda in the second-line setting.
In addition, the PBAC considered that the economic evaluation was unreliable and therefore, the cost-effectiveness estimates were highly uncertain.
The PBAC considered that the nominated place in therapy, as a second-line treatment, was incorrect as the majority of patients in the key study had failed at least two prior lines of treatment.
The PBAC considered that it would have been more appropriate to position Keytruda as a last-line treatment option. The PBAC therefore also considered that the nominated comparators were incorrect.
- July 2017 - the US Food and Drug Administration (FDA) granted accelerated approval to Keytruda for the treatment of adult and paediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) bowel cancer that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.

Lonsurf
-
July 2018 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Lonsurf on the Pharmaceutical Benefits Scheme (PBS) for the treatment of patients with metastatic bowel cancer who were treated previously or were not considered suitable for current available therapies.
The PBAC considered that in the context of limited treatment options in this disease setting, the small treatment benefit of Lonsurf may be meaningful for some patients.
The PBAC considered that the reduced price proposed, in conjunction with the proposed Risk Share Agreement, were adequate to address the residual uncertainty around cost-effectiveness and budget impact.
- March 2018 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Lonsurf on the Pharmaceutical Benefits Scheme (PBS) for the treatment of patients with metastatic bowel cancer who were previously treated with, or were not considered candidates for, available therapies including fluoropyrimidine, oxaliplatin and irinotecan-based chemotherapy, anti-VEGF therapy and anti-EGFR therapy.
- November 2017 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Lonsurf on the Pharmaceutical Benefits Scheme (PBS) for the treatment of patients with metastatic bowel cancer who were previously treated with, or were not considered suitable for current available therapies, on the basis of a modest clinical benefit, moderate toxicity and an unacceptably high and uncertain incremental cost-effectiveness ratio, since the extent of benefit observed in the trial setting may not be realised in clinical practice.
The PBAC was concerned that the financial impact of listing was substantial with a total net cost to the PBS.
- July 2017 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Lonsurf on the Pharmaceutical Benefits Scheme (PBS) for the treatment of patients with metastatic bowel cancer who were previously treated with, or were not considered suitable for, current available therapies on the basis of a modest clinical benefit, moderate toxicity and an unacceptably high and uncertain incremental cost-effectiveness ratio, since the extent of benefit observed in the trial setting may not be realised in clinical practice.
- May 2017 - the Therapeutic Goods Administration (TGA) registered Lonsurf on the Australian Register of Therapeutic Goods (ARTG), for the treatment of adult patients with metastatic bowel cancer who were previously treated with, or were not considered candidates for, fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents.
- March 2017 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Lonsurf on the Pharmaceutical Benefits Scheme (PBS) for the treatment of patients with metastatic bowel cancer who were previously treated with, or were not considered suitable for, current available therapies, based on modest clinical benefit, moderate toxicity and an unacceptably high and uncertain incremental cost-effectiveness ratio, since the extent of benefit observed in the trial setting may not be realised in clinical practice.
- November 2016 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Lonsurf on the Pharmaceutical Benefits Scheme (PBS) for the treatment of patients with metastatic bowel cancer who were previously treated with, or were not considered suitable for, current available therapies.
The decision was made on the basis of a modest clinical benefit, high and uncertain incremental cost-effectiveness ratio, and concern that the extent of benefit as observed in the clinical trial would not be released in clinical practice.
- September 2015 - the US Food and Drug Administration (FDA) approved Lonsurf for patients with metastatic bowel cancer who are no longer responding to other therapies.

Stivarga
- November 2021 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Stivarga on the Pharmaceutical Benefits Scheme (PBS), on the basis that the evidence presented indicated Stivarga is toxic and would adversely impact patients' overall quality of life, whilst having a limited impact on prognosis.
- July 2014 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Stivarga on the Pharmaceutical Benefits Scheme (PBS), on the basis that the observed improvement in comparative effectiveness associated with Stivarga was small and of uncertain clinical significance, especially in the context of the increase in serious adverse effects associated with treatment.
- November 2013 - the Therapeutic Goods Administration (TGA) registered Stivarga on the Australian Register of Therapeutic Goods (ARTG), for the treatment of patients with metastatic bowel cancer who were previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, anti-VEGF therapy, and if KRAS wild type, an anti-EGFR therapy.
- September 2012 - the US Food and Drug Administration (FDA) approved Stivarga for treatment of patients with metastatic bowel cancer who were previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, and anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.

Zaltrap
The manufacturer no longer supplies Zaltrap in Australia and it was removed from the Australian Register of Therapeutic Goods (ARTG). The ARTG is the register for all therapeutic goods that can be lawfully supplied in Australia. Sometimes a special provision is made to make available some medicines that are not listed in response to the needs of patients. To find out more visit access to therapeutic goods on the ARTG website.
- July 2013 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Zaltrap on the Pharmaceutical Benefits Scheme (PBS), on the basis of inadequate comparative efficacy and potentially worse safety resulting in an unacceptably high incremental cost-effectiveness ratio (ICER), a lack of clarity regarding the clinical place in therapy of Zaltrap, an absence of comparative data against other relevant chemotherapy regimens, and, on the basis that non-inferiority against Erbitux had not been adequately established.
- April 2013 - the Therapeutic Goods Administration (TGA) registered Zaltrap on the Australian Register of Therapeutic Goods (ARTG), in combination with irinotecan-fluoropyrimidine-based chemotherapy for the treatment of patients with metastatic bowel cancer who were previously treated with an oxaliplatin-containing regimen.
- August 2012 - the US Food and Drug Administration (FDA) approved Zaltrap in combination with a FOLFIRI (folinic acid, fluorouracil and irinotecan) chemotherapy for treatment of patients with metastatic bowel cancer that is resistant to or has progressed following an oxaliplatin-containing regimen.

Vectibix
- Mar 2018 - the Pharmaceutical Benefits Advisory Committee (PBAC) considered the report of the Drug Utilisation Sub-Committee which considered the predicted and actual use of targeted therapies for metastatic bowel cancer. The PBAC considered that the targeted therapies, including Vectibix, were being used largely as expected and recommended no further action.
- June 2017 - The US Food and Drug Administration (FDA) approved Vectibix for the treatment of patients with RAS wild type metastatic bowel cancer as first-line treatment, in combination with FOLFOX and as monotherapy, following disease progression after prior treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
- March 2015 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Vectibix on the Pharmaceutical Benefits Scheme (PBS), for the first-line treatment of patients with RAS wild type metastatic bowel cancer on a cost- minimisation basis compared with Erbitux.
- January 2015 - he Pharmaceutical Benefits Scheme (PBS) restrictions were amended for Vectibix to include only patients with RAS wild type status rather than KRAS wild type status.
- July 2014 - to align with its TGA-approved indications, the Pharmaceutical Benefits Advisory Committee (PBAC) recommended that the current PBS restrictions for Vectibix be amended urgently to include only patients with RAS wild type metastatic bowel cancer, in coordination with corresponding amends to the related MBS item description to extend mutation testing to cover all RAS mutations.
The Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Vectibix on the Pharmaceutical Benefits Scheme (PBS) as a first-line treatment option for patients with RAS wild type metastatic bowel cancer due to uncertain extent of incremental clinical benefit overs its comparators.
- March 2014 - the US Food and Drug Administration (FDA) approved Vectibix for use in combination with FOLFOX, an oxaliplatin-based chemotherapy regimen, as first-line treatment in patients with KRAS wild type metastatic bowel cancer.
- November 2013 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Vectibix on the Pharmaceutical Benefits Scheme (PBS), for the later-line treatment of patients with KRAS wild type metastatic bowel cancer.
The PBAC considered that the restrictions for Vectibix should be consistent with the current restrictions for Erbitux, and include wording so that simultaneous use of Vectibix and Erbitux or switching from one agent to the other following disease progression would not be allowed, except where patients experienced intolerance necessitating permanent treatment withdrawal.
- March 2013 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Vectibix on the Pharmaceutical Benefits Scheme (PBS) as a first-line treatment option for patients with KRAS wild type metastatic bowel cancer.
- December 2011 - the Therapeutic Goods Administration (TGA) registered Vectibix on the Australian Register of Therapeutic Goods (ARTG) for the treatment of patients with epidermal growth factor receptor (EGFR) expressing KRAS wild type metastatic bowel cancer as first-line therapy in combination with FOLFOX; as second-line therapy in combination with FOLFIRI for patients who have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan); as a monotherapy in patients after the failure of standard chemotherapy.
- November 2008 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Vectibix on the Pharmaceutical Benefits Scheme (PBS), due to uncertain clinical benefit and the resultant high and highly uncertain cost-effectiveness.
- May 2008 - the Therapeutic Goods Administration (TGA) registered Vectibix on the Australian Register of Therapeutic Goods (ARTG) for the treatment of patients with epidermal growth factor receptor (EGFR) expressing metastatic bowel cancer whose disease progressed following treatment with a fluoropyrimidine, oxaliplatin- and irinotecan-based chemotherapy.
- September 2006 - the US Food and Drug Administration (FDA) approved Vectibix following priority review for the treatment of patients with epidermal growth factor receptor (EGFR) expressing metastatic bowel cancer after disease progression on, or following treatment with chemotherapy regimens containing fluoropyrimidine-, oxaliplatin-, and irinotecan.

Erbitux
-
March 2018 - the Pharmaceutical Benefits Advisory Committee (PBAC) considered the report of the Drug Utilisation Sub-Committee which considered the predicted and actual use of targeted therapies for metastatic bowel cancer. The PBAC considered that the targeted therapies, including Erbitux, were being used largely as expected and recommended no further action.
- January 2015 - the Pharmaceutical Benefits Scheme (PBS) restrictions were amended for Erbitux to include only patients with RAS wild type status rather than KRAS wild type status.
- November 2014 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended extending Erbitux’s existing listing to include first-line treatment for patients with metastatic bowel cancer on a cost-minimisation basis compared with Avastin.
The PBAC considered that requested restrictions for Erbitux should include wording so that simultaneous use of Erbitux and Vectibix or switching from one agent to the other following disease progression would not be allowed,, except where patients experienced intolerance necessitating permanent treatment withdrawal.
- July 2014 - to align with its TGA-approved indications, the Pharmaceutical Benefits Advisory Committee (PBAC) recommended that the current PBS restrictions for Erbitux be amended urgently to include only patients with RAS wild type metastatic bowel cancer, in coordination with corresponding amends to the related MBS item description to extend mutation testing to cover all RAS mutations
- May 2013 - the Therapeutic Goods Administration (TGA) registered Erbitux on the Australian Register of Therapeutic Goods (ARTG) for the treatment of patients with epidermal growth factor receptor (EGFR) expressing KRAS wild type metastatic bowel cancer, in combination with infusional 5-fluorouracil/folinic acid plus irinotecan; in combination with irinotecan in patients who are refractory to first-line chemotherapy; in first-line combination with FOLFOX; and as a single agent in patients who have failed or are intolerant to oxaliplatin-based therapy and irinotecan-based therapy.
- June 2012 - the US Food and Drug Administration (FDA) approved Erbitux in combination with FOLFIRI (irinotecan, 5-Fluorouracil, leucovorin) for the first-line treatment of patients with KRAS wild type, epidermal growth factor receptor (EGFR) expressing metastatic bowel cancer.
- July 2010 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), for the treatment of metastatic bowel cancer in patients who meet certain criteria, on the basis of high but acceptable cost-effectiveness compared with best supportive care.
- March 2010 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), due to uncertain clinical benefit and uncertain cost-effectiveness.
- July 2009 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), due to high and uncertain cost-effectiveness.
- March 2009 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), because of high and uncertain cost-effectiveness.
- November 2008 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), because of uncertainty about the extent of clinical benefit and the resultant high and uncertain cost-effectiveness.
- October 2007 - the US Food and Drug Administration (FDA) approved Erbitux as a monotherapy for the treatment of patients with epidermal growth factor receptor (EGFR) expressing metastatic bowel cancer after failure of both irinotecan- and oxaliplatin-based chemotherapy.
- July 2006 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), due to uncertain clinical benefit and unacceptable and uncertain cost-effectiveness.
- November 2005 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), due to uncertain clinical benefit and unacceptable and uncertain cost-effectiveness.
- March 2005 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Erbitux on the Pharmaceutical Benefits Scheme (PBS), due to the uncertain extent of clinical benefit and the uncertain, but unfavourable, cost-effectiveness.
- February 2005 - the Therapeutic Goods Administration (TGA) registered Erbitux on the Australian Register of Therapeutic Goods (ARTG) in combination with irinotecan, or as a single agent, for the second-line treatment of patients with epidermal growth factor receptor (EGFR) expressing metastatic bowel cancer whose disease had progressed or was refractory to irinotecan based therapy.
- Febraury 2004 - the US Food and Drug Administration (FDA) approved Erbitux in combination with irinotecan, or alone, for the treatment of patients with epidermal growth factor receptor (EGFR) expressing metastatic bowel cancer.

Avastin
The manufacturer no longer supplies Avastin in Australia and it was removed from the Australian Register of Therapeutic Goods (ARTG). The ARTG is the register for all therapeutic goods that can be lawfully supplied in Australia. Sometimes a special provision is made to make available some medicines that are not listed in response to the needs of patients. To find out more visit access to therapeutic goods on the ARTG website.
- March 2018 - the Pharmaceutical Benefits Advisory Committee (PBAC) considered the report of the Drug Utilisation Sub-Committee which considered the predicted and actual use of targeted therapies for metastatic bowel cancer. The PBAC considered that the targeted therapies, including Avastin, were being used largely as expected and recommended no further action.
- November 2014 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended amending Avastin’s existing listing to include second-line treatment for patients with RAS wild type metastatic bowel cancer after failure of first-line anti-EGFR antibodies.
- July 2008 - the Pharmaceutical Benefits Advisory Committee (PBAC) recommended the listing of Avastin on the Pharmaceutical Benefits Scheme (PBS), on the basis of high, but acceptable cost-effectiveness.
- March 2008 - the Pharmaceutical Benefits Advisory Committee (PBAC) rejected the listing of Avastin on the Pharmaceutical Benefits Scheme (PBS), on the grounds of a high cost effectiveness ratio in the first-line population and high and uncertain cost-effectiveness in the second-line population.
- February 2005 - the Therapeutic Goods Administration (TGA) registered Avastin on the Australian Register of Therapeutic Goods (ARTG), in combination with fluoropyrimidine-based chemotherapy, for the treatment of patients with metastatic bowel cancer.
- February 2004 - the US Food and Drug Administration (FDA) approved Avastin, in combination with intravenous 5-fluorouracil-based chemotherapy, for the first- and second-line treatment of patients with metastatic bowel cancer.
1. Stollznow. 2014. My Cancer My Voice Bowel Cancer Patient Survey. April 2014.
2. Wonder Drug Consulting. 2014. Reimbursement success rates and timelines for new medicines for cancer; and international comparison. Available at http://medicinesaustralia.com.au/files/2013/07/140323_OIT_Wonder-Report_FINAL.pdf
3. Vickers M (2013). Slow and steady: incremental survival improvement in advanced colorectal cancer. OE 2013: 12,1.